Air quality
Air
Air is a mixture of several gases making up the atmosphere, the protective layer that envelops our planet. It filters the sun's rays with the ozone layer and keeps a certain amount of heat that is necessary for life on earth, thanks to the greenhouse effect. The atmosphere is composed of 78% nitrogen (N2), 21% oxygen (O2), and a series of other gases (Ar, CO2, etc.).
It is this combination of gases in these precise proportions that allows life on our planet. Too much nitrogen or too much oxygen would be fatal for most living species today, as would too little of these gases. However, this was not always the case: five billion years ago, the atmosphere was made up of 80% water (H2O) and 20% carbon dioxide (CO2). This atmosphere, dubbed "primitive", did not have oxygen (O2), an essential element for life. It is after various long and complex processes that the Earth's atmosphere has become into what we know it to be today.
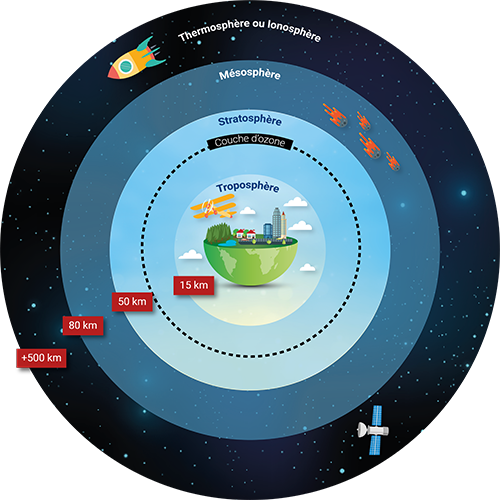
The Earth's atmosphere is made up of different layers stacking up over a distance of about 500km on the ground. The atmospheric layer in which we live, the troposphere, occupies a thickness of less than 20km but represents 80% of the mass of the atmosphere and contains gases and particles of anthropogenic origin.
The home of meteorological phenomena, it is characterised by an average decrease in temperature with an altitude of about 6.5°C/km. However, the vertical temperature profile varies over time and depending on location. The diurnal cycle in temperate regions induces night-time cooling of the soil and heavier air near the soil. This phenomenon stabilises the lower layers of the troposphere, particularly in the event of temperature inversion. The dispersion of pollutants is then reduced, or even made impossible, which results in a degradation of air quality. On the contrary, the ground heats up on a sunny day, then heats the air in contact with it, thus generating upward movements favourable to a good dispersion of pollutants.
The troposphere is located below the stratosphere, characterised by a significant absorption of ultraviolet rays by the ozone. The stratosphere plays a key role since it protects us from UV radiation. This is the reason why CFC (chlorofluorocarbon) gases were banned in the 1980s: they destroyed the ozone layer to the point of creating the Antarctic ozone hole. The absorption of UV rays by the ozone also has the consequence of making it a particularly stable layer: the temperature in the stratosphere increases from the bottom to the top, from -60°C at the level of the tropopause to -3°C at the level of the stratopause, 50km from the ground.
Air quality
A pollutant is defined as any substance present in the ambient air liable to have harmful effects on human health and/or on the environment as a whole. Air quality describes the level of pollutants.
To better understand this, we can draw an analogy with health.
Being in good health is not just about not being affected by a disease that can affect different organs or parts of the body. Indeed, one can consider oneself in good health when the whole body is functioning properly.
Likewise, for air quality to be qualified as good, all pollutants must be found at levels such that there is no impact on health and/or on the environment.
This is why it is so difficult to answer the question relating to the evolution of air quality. Indeed, some parameters can evolve favourably, and others not. In addition, scientific knowledge, and toxicological knowledge in particular, is constantly evolving with updates of the reference thresholds (guide values) and increasing attention being paid to new pollutants.
To make information accessible to the general public, air quality is often synthesised by an index. There are several methodologies for defining this index, but all of them use a small number of pollutants measured in real time and are based on short-term health effects. In Belgium, the name of the ambient air quality index is BelAQI, which stands for Belgium Air Quality Index.
Air quality assessment
Air quality assessment is any method used to measure, calculate, predict or estimate the concentrations of pollutants or their deposits on the ground. The assessment of air quality is therefore carried out by a set of complementary methods, of which measurement is only one of the components.
To measure air quality, we have a set of equipment, and for each pollutant or family of pollutants, the methods implemented are different and grouped into measurement networks.
There are two main families of networks according to their mode of operation:
-
the real-time measurement network (also called “alert network”);
-
deferred measurement networks, where a sample is taken in the field and then analysed a posteriori in the laboratory.
In the real-time measurement network, a series of monitors continuously measure pollutants. These monitors are linked to an acquisition system and every half an hour, the results are transmitted to a centralised computer system. This measurement network can be considered as the capital element of measurement systems because it was designed as the alert network capable of providing information, in real time and continuously, with the aim of protecting the health of the population.
To ensure information is provided to the public, the results are published on this website. The real-time measurement network currently consists of more than 80 monitors spread over 24 stations, 8 of which are also equipped with meteorological sensors. The pollutants measured are sulphur dioxide (SO 2), nitrogen oxides (NOx), ozone (O3), carbon monoxide (CO), particulate matter (PM10 and PM2.5), and black carbon (BC).
It is not always technically possible to measure pollutants continuously and, therefore, deferred measurement networks complete the system.
These so-called “deferred” networks are all based on the same principle: the pollutants contained in the air are captured either on an absorbing phase (tubes with specific adsorption phases, foams, solution) for the gaseous pollutants, or on a filter for solid pollutants. It is this phase or this filter which, once brought back to the laboratory, is analysed. Various pollutants are assayed within these networks: metals, volatile organic compounds (VOCs), polycyclic aromatic hydrocarbons (PAHs), fluorine, nitrogen dioxide, ammonia, etc.
In Wallonia, there is thus a network for measuring metals in particulate matter, a network for VOCs, a network for measuring PAHs, and, finally, a network for measuring fluorides.
Finally, in the “Sedimentable dust” and “acid fallout” networks, we no longer measure the quantity of pollutants present in the air, but their deposition on the ground (dust, metals, etc.).
Alongside the permanently-installed measurement stations, Wallonia has a set of equipment that can be installed wherever the need for measurement is felt and for a fixed period of time. The objectives of these campaigns are manifold: respond to local pollution problems, provide additional information to fixed networks, carry out a preliminary study before the final installation of a measurement point, study a particular environment, etc.
ThePublic Service Scientific Institute (ISSeP) is responsible for operating these networks on behalf of AwAC (Walloon Air and Climate Agency). A general presentation video is available on ISSeP Web TV.
It is useful to specify that ISSeP is also active with regard to the measurement of ultra-fine particles (UFP), the measurement of certain compounds by the use of passive tubes, and in the development and the fine-tuning of a low-cost platform made up of microsensors allowing the analysis of certain “conventional” gases such as nitric oxide, nitrogen dioxide, ozone and fine particles of the PM2.5 type.
Regulatory context and benchmarks
Assessing air quality is only the first step. Afterwards, it is still necessary to compare the levels obtained with reference values to judge the possible impact of pollutants on health and/or the environment.
Europe has put in place legislation aimed at:
- setting ambient air quality objectives in order to minimise the negative consequences for both health and the environment;
- assessing air quality in a common way for all states at a European level;
- obtaining information on air quality in order to help control air pollution;
- making information available to the public;
- maintaining air quality where it is good and improving it in other cases;
- promoting cooperation between countries with a view to reducing atmospheric pollution.
European directives thus define the means to be implemented to assess air quality, impose constraints on thresholds not to be exceeded, and set air quality objectives.
Currently, two directives apply. Directive 2008/50/EC regulates sulphur dioxide, nitrogen oxides, ozone, carbon monoxide, particulate matter(PM10 and PM2.5), benzene and lead, while directive 2004/107/EC regulates arsenic, cadmium, nickel and PAHs. Wallonia has transposed these directives into its legislation in order to make these regulations directly applicable.
Besides the legal values to be respected, there are other sources of reference values. The most important is, without a doubt, the World Health Organization (WHO) which defines guide values for a series of pollutants. It should be noted that unlike European directives, only the health criterion is taken into account in establishing the thresholds and, therefore, the WHO criteria are often more severe.
We can also turn either to foreign legal values, or to values published by reputable organisations. Thus, the Walloon Air and Climate Agency (AwAC) has compiled data from different sources to propose quality and intervention criteria for a much larger set of substances.
In the event that the values were to exceed the values authorised in the directives, AwAC is responsible for proposing to the authorities (Walloon and European) a strategy of operational corrective measures aimed at correcting the situation.
To find out more, visit the dedicated section
The climate
In addition to atmospheric pollutants, gaseous or particulate, which have harmful effects on health, the troposphere contains various gases which give it the properties of a greenhouse: the ground is heated by absorption of the part of the solar radiation that passes through all the layers of atmosphere; then, in turn, it re-emits infrared radiation, which is absorbed into the troposphere. Thanks to the greenhouse effect of water vapour and carbon dioxide, we know there is an average temperature of 15°C at the earth's surface, instead of -18°C. However, CO2 emissions initiated around 1950 by industrial development continually increase the concentration of this greenhouse gas in the troposphere. The level of CO2 has increased by more than 30% in 70 years, which is considered, on a time scale of major climatic phenomena, as an increase of very short duration. The global warming caused by this sudden increase risks translating into major local upheavals.







